In terms of plant growth, the pH of the soil the plant is growing in can have a massive impact on yield, so it is important to optimize the pH in the soil.
Solutions shown to be 7 on the pH scale are neutral, so pure water would be neutral.
Solutions under 7 on the pH scale are acidic – cider vinegar, for example, is around 4 on the scale and stomach acid (hydrochloric acid) is around 2-3.
Solutions over 7 on the pH scale are alkaline – bleach is around 13 on the scale and a baking soda solution is about 9.
Testing the pH of a Solution
There are a number of ways to test the pH of a solution. It’s a case of how accurate you need to be. In terms of growing plants, you need more of a ballpark than precision. The following methods can be used to test pH to varying degrees of accuracy. In terms of when the SHTF is, you should prepare by putting aside some of the commercially available methods and then use the natural method when you run out.
Litmus paper:
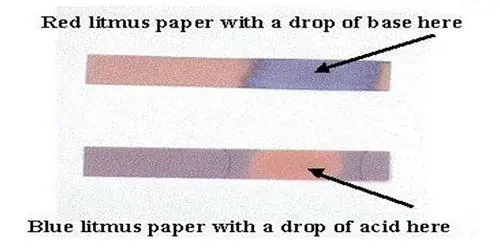
This is a commercially available method and actually a very old technique for gauging pH. Litmus paper is a book of paper sticks, imbued with dyes taken from lichen plants. There are two types: Blue litmus and red litmus. Each is used to test for the corresponding acid or alkaline.
Blue paper —-à (becomes) red in acid
Red paper —-à (becomes) blue in alkaline
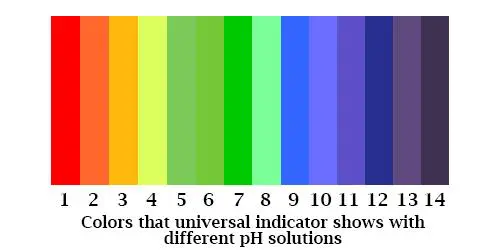
Universal indicator: This comes in two forms, paper, and a liquid. Unlike Litmus, it covers the whole pH spectrum in one go. It is made up of a mix of chemical components, which show a color change under different pH conditions. In the paper form, which often comes in the form of a box of sticks, the color change varies across the spectrum from red to purple. You wet the stick with your solution then hold the stick against a chart on the box, which gives you the pH of the solution you are testing. The solution version of the universal indicator acts in a similar way, changing color depending on the pH.
Natural pH Indicator
If you don’t have any commercial pH indicators to hand, you can get a general idea of soil pH by using natural substances. Here are a few ideas:
- Beet juice will change from red to blue/purple if you add an alkaline solution.
- Baking soda will fizz if you add an acidic solution to it.
- Red cabbage boiled up in water – the water will show a color change from red (acid), to purple (neutral), through to green/yellow (alkaline) when you add a solution to it.
Testing the pH in Soil
To test the pH of your soil, add some soil to a vial. Add some water and give it a good shake. Leave it to stand for a few minutes, letting the soil settle. Use the water (decanted) to test the pH.
Plants and pH
The reason that pH is often crucial in plant growth is because the pH in soil affects how well certain nutrients dissolve in it. In other words, pH directly affects solubility. So getting the pH of your soil solution right can greatly affect the yield of plants, such as fruit and vegetables.
The problem is that soil pH is affected by the environment. For example, if the rain in your area is very acidic, i.e. has dissolved sulfur compounds in it, or if you have water contaminated by ions, like potassium and magnesium, then the subsequent pH in the soil will be affected.
We also see very acidic soils where there has been intensive farming that uses commercial fertilizers. It may be that we inherit some pretty poor soil to work our magic from.
There are 13 important elements needed to keep plants healthy and get good yields. These nutrients need to get taken up into the plant after being dissolved into the soil in solution form. So we need to make sure that they dissolve and low pH soils can adversely affect the solubility of these crucial elements.
How to Adjust the pH in Soil
The best soil pH is around neutral, so we’re talking in the 6.5-7.5 range. At this level of acidity, the nutrients plants need to grow are more available to them as they dissolve optimally. Low acidity can cause nutrients, like calcium and magnesium to become too soluble and they end up being leached away from the plants. Once you get into the alkaline zone, nutrients like phosphorus, iron, and manganese get locked up into other compounds and the plants can’t access them.
If you test your soil pH and it is outside that neutral zone you can make adjustments to get it back inside it.
NOTE: Don’t adjust the pH of fertilizers, compost, or manure.
Soil Too Acidic (pH in soil below 6)
If your soil falls under pH 6 you will need to add an alkaline material to adjust it back up into the neutral range. The most common method of doing this is to ‘lime’ the soil. Garden lime is calcium carbonate, which is an alkaline. You can buy this as a commercial substance, or you can alternatively use calcified seaweed or ground chalk. Another version of garden lime is dolomite lime, which contains magnesium. This type of lime is used if you know you need extra magnesium in your soil.
The best time to add this to the soil is in the winter before planting out. Dig half the lime into the soil and sprinkle the rest over the top.
You can work out how much to add by running your own tests.
- Take a portion of pH-tested soil in a bucket.
- Add a small amount of the lime to the soil and dig it in (take note of the amount of lime as you’ll need it later).
- Now re-test the pH of the soil
- If it’s still acidic, add a little more lime; if it’s too alkaline do the test again, using less lime
- Once you have your amount of lime added to get the neutral pH you can approximately calculate how much you’ll need for your planting area.
As a rule of thumb, if your original soil pH is 5.5 you’ll need around 25 ounces per square meter of soil, but this is also dependent on the soil type, e.g. sandy, chalky, etc.
Soil Too Alkaline (pH in the soil above 7)
There are a few products you can use to adjust the pH in soil downwards:
Sulfur
Adding sulfur to the soil to acidify it is a slow process. I’ve used this method to grow bilberries, which are a plant that actually likes acid soils. Normally you wouldn’t want to make the soil as acid as that needed by bilberries, but the principle is the same. To acidify the soil, add finely ground sulfur, missing it into the soil. Sulfur takes time to become active, as bacteria need to convert it first. Also, use it in the warmer months, as winter will slow down any bacterial activity.
Aluminum Sulfate
This needs small amounts to make a big pH change, so use sparingly and carefully. If you end up with acidic soil, you’ll lose nutrients.
Iron Sulfate
This has the same effect as aluminum sulfate but you need larger amounts to get the same pH change. Use it with a phosphate fertilizer as it can leach phosphate.
You can use the same type of test shown above, to work out how much of the acid compound to apply to your soil. But in general, adding sulfur will take a long time to take effect so be prepared for this.
You may also like:
How to Keep Eggs Fresh for Months with Mineral Oil
Do you know why you should never put a tall fence around your house? (Video)
10 Bugs You Should Never Kill In Your Garden
How To Install A Trip Wire Alarm On Your Property
If You Have This Plant in Your Backyard, You Will Never Run Out of Soap